Research
In plant tissue, cells are cemented together via rigid (passive) cell walls, thus prohibiting cell migration. This also makes plant morphogenesis slow in dynamics, which contributes toward a morphology that consists of a limited set of structures during embryogenesis. Most of the organs we commonly observe are formed during post-embryonic development. Plants continuously generate new organs during their post-embryonic growth. The cells responsible for this process are located at the opposite ends of the plant body axis, known as the shoot and root meristems. Stem cells are confined to these meristems and act as a reservoir for the cells needed to form root and shoot-derived organs. These stem cells not only provide cells during normal development but also play a crucial role in tissue restoration following injury. Our laboratory studies the mechanisms of shape generation during development and regeneration. We use Arabidopsis as a model to investigate shoot and root morphogenesis. We simulate fundamental cellular behaviors to provide strategic guidance for the experimental search for mechanisms underlying plant morphogenesis. Our current projects explore how mechanical forces experienced by cells activate biochemical cues (gene activity or hormone signal) to guide morphogenesis.


Fig 1: regenerating shoot primordial marked by membrane localized protein

Fig 2: re-establishment of the regenerating root tip. Bright spots are fluorescently labeled PLT2 protein
In animal tissue, cells are connected via flexible (active) cell membranes, which allow the exchange of cell neighbors, thus facilitating cell migration. This allows animal morphogenesis to be very dynamic. During animal development, collective cell migration is an essential process for tissue formation or wound repair, where the dynamics of a collection of cells are coordinated by genetic as well as geometric cues. The directed cell movement requires cells to be spatially informed through cell polarization. Studies on the budding Yeast have shown that changes in cell shape destabilize the polarization domain, and additional mechanical feedback is necessary to stabilize the polarity against the geometric effect. However, in Drosophila, cells undergo continuous changes and still, the stability of cell polarity remains robust. These observations indicate the presence of a regulatory mechanism that provides stability to the polarity against the changes in cell shapes. To this end, we develop multi-scale modeling frameworks to simulate the complex coupling between cell shape and polarity. Further, we use Drosophila epithelium as an experimental system to test the model predictions. Together, our current project aims at uncovering feedback control of robust cell polarity that drives robust epithelial morphogenesis.


Fig 3: wound healing through cell migration
heterogeneity in cell polarity

generation of contractile forces

cell migration
Modeling approaches
Primarily, we use three different types of modeling approaches. When small-scale details of cellular processes need to be considered, we choose the vertex modeling approach. In this modeling approach, a cell shape is described by a collection of connected nodes (polygon). The configuration of cells/tissue is determined by the mechanochemical state of the individual nodes. When small-scale details of cellular processes are not necessary to be considered, we opt for the continuum modeling approach in which cell/tissue is described as a continuous entity. This modeling approach allows for the definition of the mechanochemical state of any position. An extension of the continuum modeling approach is the finite element approach (FEM), which is adapted when cell/tissue shape cannot be described as a continuous entity, instead approximated by a triangular mesh. Also, we develop novel image analysis tools through custom Python scripts to extract meaningful information from the experimental data that can be used in modeling. We use image segmentation programs such as MorphoGraphX (MGX) and Tissue-Analyzer for image analysis.
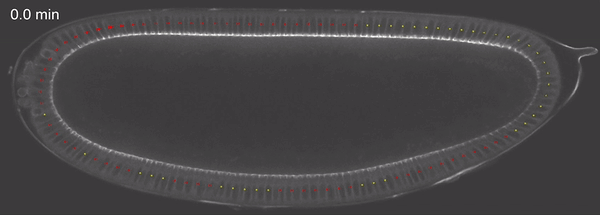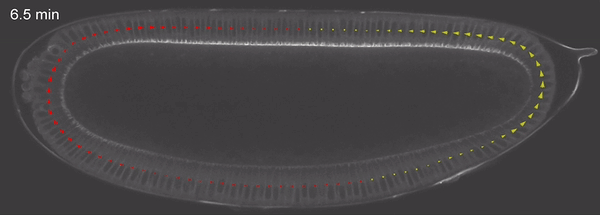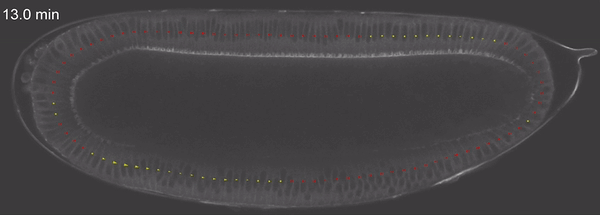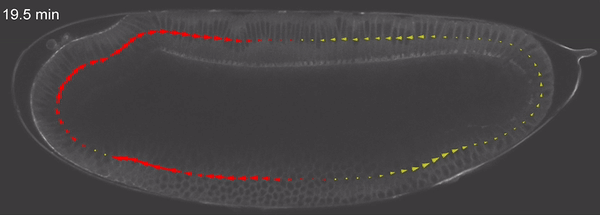
Fig 4: tissue velocity (clockwise, anticlockwise) quantified using PIV
substrate
myosin

vertex model
continuum model
midline

plant leaf